The search for new pharmaceutical drugs by medicinal chemists relies on the synthesis of diverse compound libraries for biological testing. Of the reactions used for this purpose, those involving boron-containing organic molecules (known as organoboron compounds) are among the most popular because of the commercial availability and wide reactivity of these reagents1. Writing in Nature, Kim et al.2 report a method for the synthesis of organoboron compounds called borylated azines. The authors demonstrate that these compounds can participate in reactions common to other organoboron compounds, but offer distinct advantages, such as ease of preparation and impressive stability. Importantly, these reagents will allow a full exploration of the therapeutic potential of molecules that have previously been difficult to prepare.
Azines are nitrogen-containing analogues of benzene rings and are present in many of the top-selling pharmaceuticals approved by the US Food and Drug Administration (see go.nature.com/2dirpwf). These medicines target several disease areas, including arthritis, diabetes and several types of cancer. If a boron-containing group is attached to an azine, the resulting borylated azine can be used as a reagent for the synthesis of many different azine-containing molecules — a crucial process for diversifying compound libraries. Kim et al. explored the use of transformations called C–H functionalization reactions to prepare borylated azines.
Although once regarded as an academic curiosity, C–H functionalization reactions are now a powerful methodology in organic synthesis. In these processes, a carbon–hydrogen (C–H) bond is converted into a carbon–X bond, where X can be any atom other than hydrogen. C–H functionalization can greatly streamline a synthetic procedure by reducing the number of steps it takes to get to a specific target molecule.
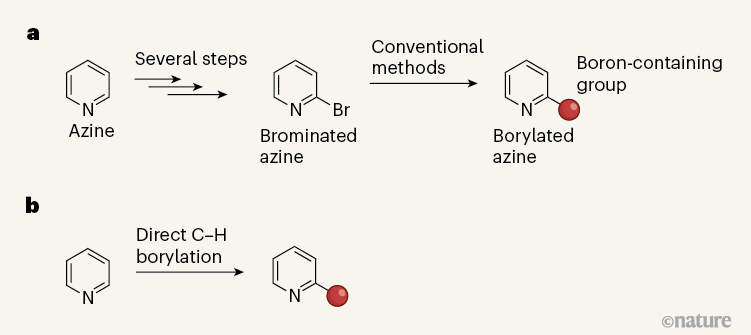
Borylated azines are typically synthesized from their bromine-containing analogues (Fig. 1a). But although these brominated starting materials are commercially available, they are more costly than the equivalent azines that lack a bromine atom. For example, 2-bromopyridine can be about 25 times more expensive per mole than pyridine, which reflects the cost of the synthetic steps needed to attach a bromine atom to pyridine3.
A C–H functionalization reaction that directly introduces a boron-containing group to an azine can bypass these steps, saving valuable time and resources. If the conventional synthetic approach is like a bus route that has multiple transfers, C–H functionalization is like a non-stop express train. But before boarding this train, it is crucial to make sure that it stops at your desired destination: azines often have several C–H bonds, which means that synthetic chemists must devise clever strategies to ensure that only the desired C–H bond reacts, rather than another.
The development of iridium-catalysed borylation methods was a notable advance in the field of C–H functionalization4. With azines, however, iridium-catalysed borylation occurs at a C–H bond distal to the nitrogen atom in the azine ring, mainly at the β-position (the second-nearest position to the nitrogen). These methods cannot provide products with a boron group adjacent to the nitrogen (the α-position), which, in turn, means that the borylated products cannot be used to make molecules in which the azine ring is connected at the α-position. This is a notable limitation, because many drug molecules contain azines attached at that site.
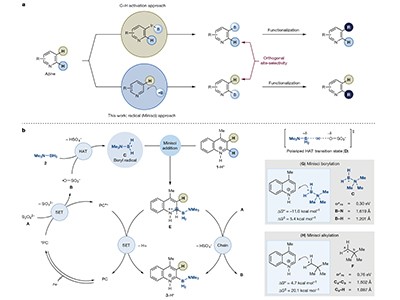
Kim and colleagues have now solved this problem by taking advantage of the inherent reactivity of azines towards radicals — atoms or molecules that contain an unpaired electron. This reactivity was heavily explored by the chemist Francesco Minisci in the 1960s with ‘alkyl’ radicals, which have the unpaired electron centred on a carbon atom. In the presence of acid, carbon-centred radicals react with azines predominantly at the α-position, leading to attachment of a carbon atom to that site and loss of a hydrogen atom5.
In their work, Kim et al. show that boron-centred radicals can react similarly, allowing boron-containing groups to be installed at the α-position and providing products that currently cannot be made using iridium-catalysed reactions (Fig. 1b). If the reactive α-sites are blocked by attached chemical groups, borylation can occur at other positions around the azine ring. The authors’ key discovery was that an amine-borane reagent (Me3N·BH3, where Me is a methyl group, CH3), in the presence of an organic catalyst, acid and light, can form boron-centred radicals with the necessary reactivity for Minisci-type chemistry.
An added benefit of Kim and co-workers’ chemistry is that it installs an amine-borane group (BH2-NMe3) in the azine. The amine portion of the group (NMe3) confers stability on the borylated azine — without it, the boron atom has an incomplete shell of valence electrons and could undergo reactions that lead to the compound’s decomposition. Indeed, the authors’ borylated azines are stable enough to be stored in ambient conditions for several months, without the need for specialized storage.
Stable compounds can be unreactive, especially if the site of reactivity is blocked off — and in this case, the boron centre is blocked off by the attached amine group. Chemists might therefore be wondering whether further reactions of the borylated azines are possible. The answer is yes: these compounds can participate in oxidations, and in various ‘cross-coupling’ reactions to form other types of bond (such as carbon–carbon, carbon–nitrogen and carbon–oxygen bonds), providing entry to medicinally relevant molecular scaffolds. The yields of some of the cross-coupling reactions are modest, but α-borylated azines are generally challenging substrates for cross-coupling, owing to their tendency to decompose through protodeboronation (replacement of the boron group with a hydrogen).
It would now be useful to know whether these borylated azines can participate in reactions in which the amine-borane group is replaced with a fluorine atom6. Fluorine-containing groups are valuable motifs in medicinal chemistry, and therefore having access to fluorinated azines could be transformative in drug discovery. Moreover, if the authors’ chemistry can be used to incorporate fluorine into molecules as the final step of a synthesis, it might be possible to use it to label pharmaceutical compounds with a radioactive fluorine-18 atom — thereby allowing the compounds to be visualized in the body using positron emission tomography imaging7. Finally, given the balanced stability and reactivity profile of Kim and colleagues’ borylated azines, it will be interesting to see whether unique synthetic applications emerge for these reagents, particularly in situations for which conventional organoboron compounds fall short.
"direct" - Google News
July 26, 2021 at 05:34PM
https://ift.tt/3zGaj1B
Direct installation of boron groups offers boost to medicinal chemistry - Nature.com
"direct" - Google News
https://ift.tt/2zVRL3T
https://ift.tt/2VUOqKG
Direct
Bagikan Berita Ini














0 Response to "Direct installation of boron groups offers boost to medicinal chemistry - Nature.com"
Post a Comment